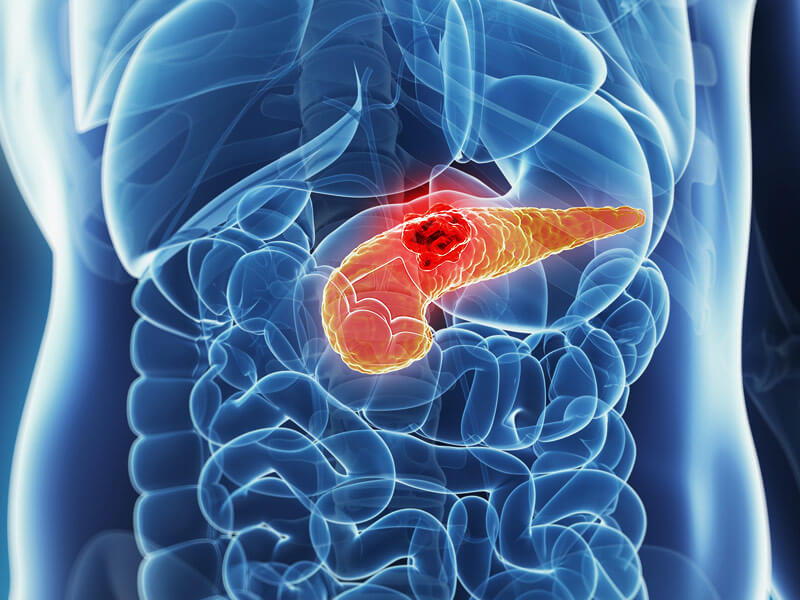
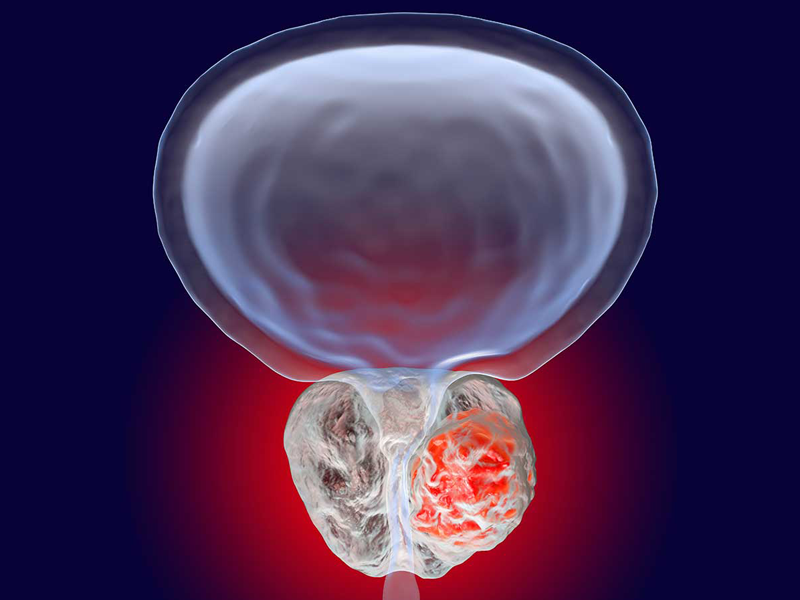
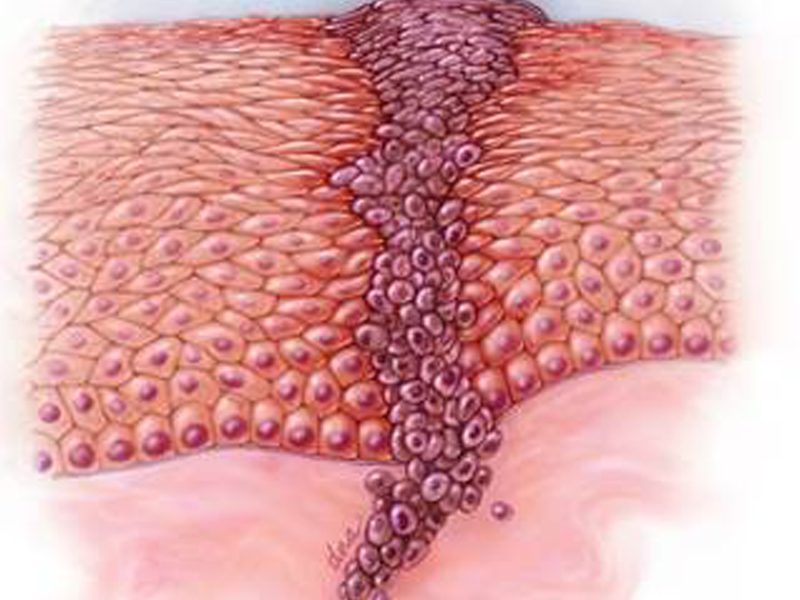
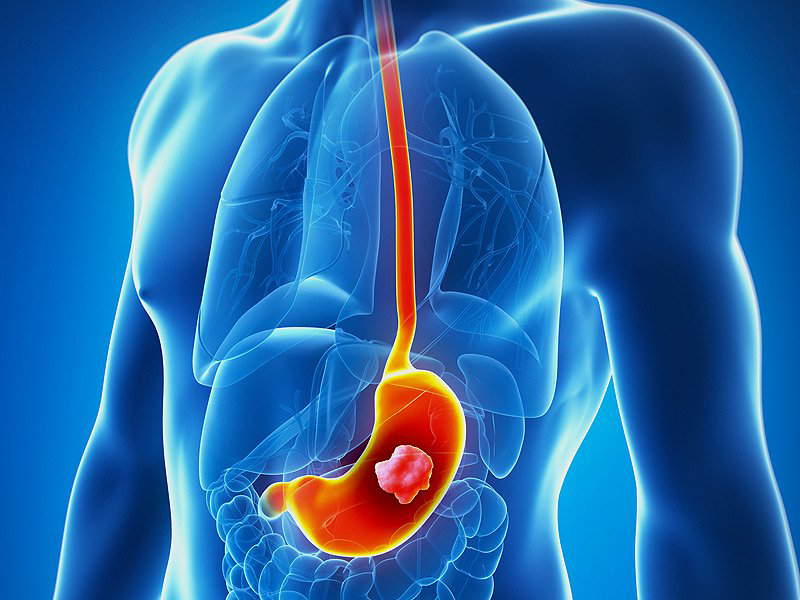
Dendritic Cell based Therapeutic Cancer Vaccine is approved by the US-FDA for Prostate Cancer and Indian FDA for Prostate, Ovarian, Colorectal and Lung Cancer.
Infrastructure
We have state of art clinical and stem cell laboratory facilities compliant with local regulatory requirements in India and Trinidad. Novo stem cell laboratories in India and Trinidad are hi-tech cellular processing clean room facilities equipped with cell sorters, FACS, confocal microscopes, incubators, controlled rate freezers, class 100 biosafety cabinets and cryostorage containers.
Why Choose NOVO Cellular Medicine Institute?
 5+ years of experience
5+ years of experience
 Team of renowned Clinicians and Scientists
Team of renowned Clinicians and Scientists
 World class clinical and laboratory facilities
World class clinical and laboratory facilities
 Our research has been published in international journals
Our research has been published in international journals
 We treat various types of cancers / disorders
We treat various types of cancers / disorders
 Most economical and effective treatment
Most economical and effective treatment
 Pioneers for Immunotherapy in the Caribbean region
Pioneers for Immunotherapy in the Caribbean region
Cancers We Treat
Patients Testimonials
The Amazing Clinic! Wonderful Support!
All during the Immunotherapy i was able to go to work as usual. There were no feelings of sickness, nausea, hair loss or any such thing associated with other treatments. There were no side effects and i continued life as normal.I was happy that i chose Immunotherapy. I would recommend this to anyone suffering with cancer. Read more....
Get In Touch With Us
Please feel free to contact Dr. Chandan on +1 (868) 3482702 /7065442 between 10 am to 6 pm between Monday to Friday.
Outside working hours, please leave your details below for a call back.