Immunotherapy is type of treatment in which certain cells of a person’s immune system are isolated. Separated cells grown to stimulate own immune system to work harder or smarter to attack cancer cells. Dendritic cell have been recognized as most potent antigen presenting cells that capable of activating T cells against tumor antigen. Immunotherapy using dendritic cells has emerged as a potent option for the treatment of the solid tumours. Over the past few decades, multiple clinical trials have been carried with the aim of increasing the efficacy of this therapy. Many protocols have been developed to generate large scale production of dendritic cell which have been used in clinical trials. Dendritic cell therapy has demonstrated high overall response rates, resulting in tumor regression and prolonged survival. Due to unique characteristics of the dendritic cell, Immunotherapy using dendritic cell therapy has emerged as one of the potent treatment option for cancer patient.
In 1973, Rockefeller University scientist Ralph M. Steinman identified the cell type that is almost singularly responsible for commanding the efforts of all other immune cells: the dendritic cell. For this discovery, Dr. Steinman received the 2011 Nobel Prize in Physiology or Medicine. Dendritic cells are a type of antigen-presenting cell (APC) that form an important role in the adaptive immune system. The main function of dendritic cells is to present antigens and the cells are therefore sometimes referred to as “professional” APCs. In addition, only the dendritic cells have the capacity to induce a primary immune response in the inactive or resting naïve T lymphocytes. To do this, the dendritic cells capture the antigens from invading bodies, which they process and then present on their cell surface and presented, along with the necessary accessory or co-stimulation molecules. Based on the emerging concept of the central role of DC in the initiation of immune responses, DC vaccines have now reached clinical testing. Recently, a number of groups have used these cells as immunotherapeutic agents in the treatment of infectious diseases and human tumors. First studies of DC vaccination for cancer immunotherapy in humans have already been published. These early pilot trials have established the general safety and feasibility of this approach, in addition to demonstrating clinical activity for several tumor types. Further clinical trials of DC vaccination are under way involving more than 1000 patients around the world. These studies include a broad spectrum of tumours: colorectal and lung carcinoma, renal carcinoma, prostate cancer, Ovary cancer, breast cancer, multiple myeloma and multiple cancers. In summary, these studies have shown that DC vaccination may elicit tumor regression even in advanced cancer and have demonstrated the general safety of DC vaccination in cancer patients.
After administration of mature dendritic cells in the cancer patient, dendritic cell will migrate to lymphoid organ. This will results in generation of tumor specific T cells. Activated T cells will migrate to cancer cells and destroy/inhibit the cancer cells.
For clinical implementation of DC therapy; valid technique for generation of clinical grade DC processing defined phenotype and functional characteristics need to be developed. Peripheral blood monocytes represent most common source for in vitro DC preparation. Large number of DC can be generated by invitro differentiation of peripheral blood monocytes which are isolated from whole blood or leukapheresis products by density gradient centrifugation and adherence. The differentiation of monocyte into dendritic cells requires cytokines like GM-CSF and IL-4. The immature DC can be further mature by adding mature DC by adding maturation stimulus. Quality control of the clinical grade of the DC preparation should include DC yield; purity; viability; cryopreservation as well as phenotype and functional parameters. In the last decade, a large number of clinical trials were carried out to establish the therapeutic efficacy of DC vaccines. Among the over 200 DC based clinical trials so far, solid tumors were the most common cancer treated which established the feasibility and safety of DC vaccines.
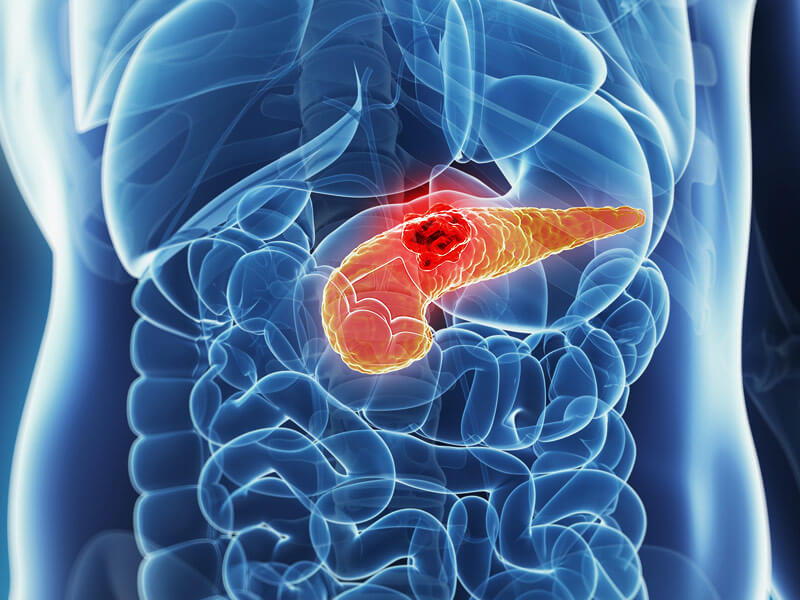
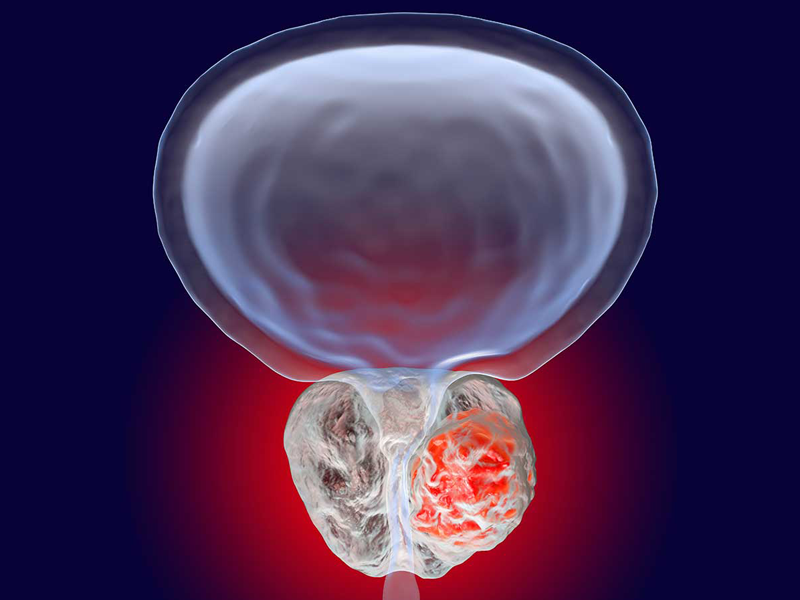
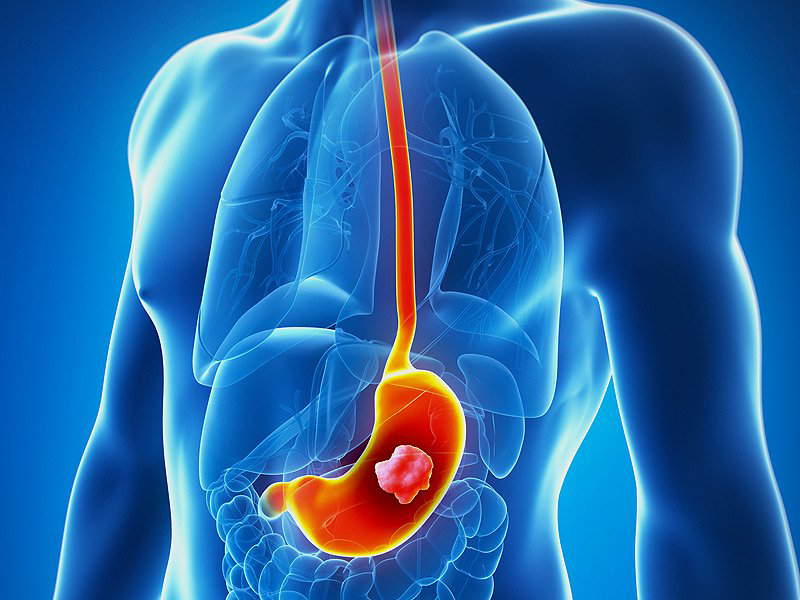
Dendritic Cell based Therapeutic Cancer Vaccine is approved by the US-FDA for Prostate Cancer and Indian FDA for Prostate, Ovarian, Colorectal and Lung Cancer.
Infrastructure
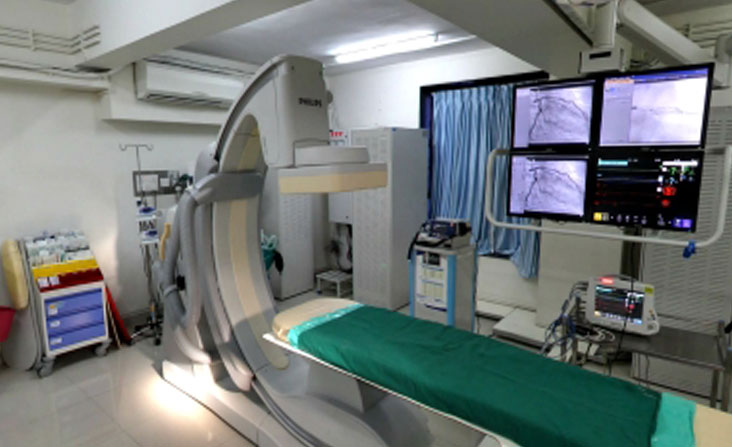
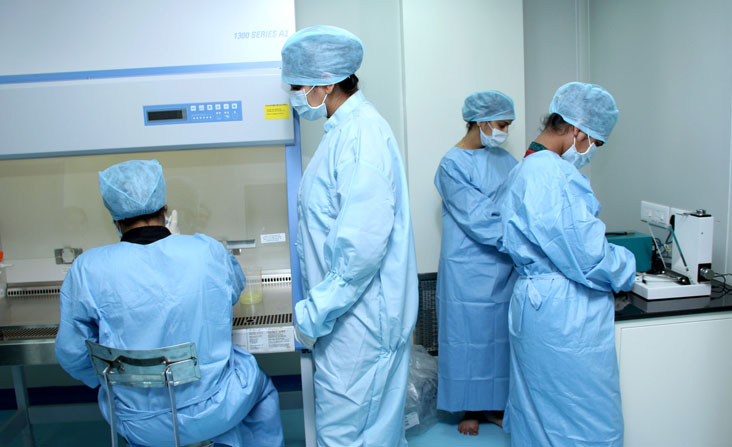
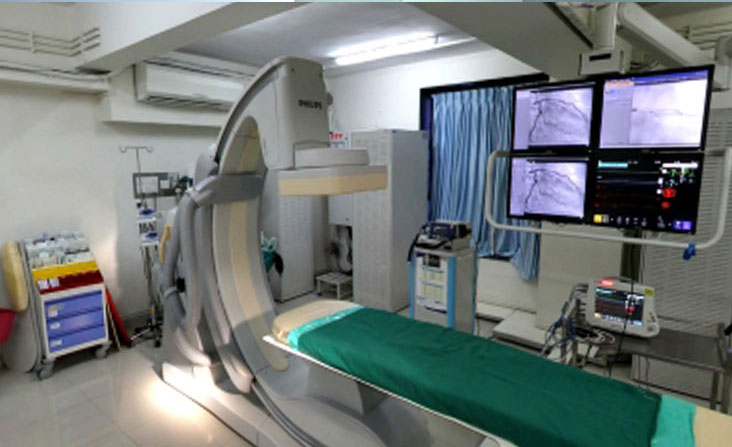
We have state of art clinical and stem cell laboratory facilities compliant with local regulatory requirements in India and Trinidad. Novo stem cell laboratories in India and Trinidad are hi-tech cellular processing clean room facilities equipped with cell sorters, FACS, confocal microscopes, incubators, controlled rate freezers, class 100 biosafety cabinets and cryostorage containers.
Why Choose NOVO Cellular Medicine Institute?
 5+ years of experience
5+ years of experience
 Team of renowned Clinicians and Scientists
Team of renowned Clinicians and Scientists
 World class clinical and laboratory facilities
World class clinical and laboratory facilities
 Our research has been published in international journals
Our research has been published in international journals
 We treat various types of cancers / disorders
We treat various types of cancers / disorders
 Most economical and effective treatment
Most economical and effective treatment
 Pioneers for Immunotherapy in the Caribbean region
Pioneers for Immunotherapy in the Caribbean region
Cancers We Treat
Patients Testimonials
The Amazing Clinic! Wonderful Support!
All during the Immunotherapy i was able to go to work as usual. There were no feelings of sickness, nausea, hair loss or any such thing associated with other treatments. There were no side effects and i continued life as normal.I was happy that i chose Immunotherapy. I would recommend this to anyone suffering with cancer. Read more....
Get In Touch With Us
Please feel free to contact Dr. Chandan on +1 (868) 3482702 /7065442 between 10 am to 6 pm between Monday to Friday.
Outside working hours, please leave your details below for a call back.